An ensemble of four structures between which a membrane protein rapidly switches underlies a critical system for passing messages across the cell membrane.
Anton Reveals Ensemble of Four Rapidly Interchanging Structures that Suggests Purpose to the Chaos as Well as Potential Drug Targets
GTPases are a family of proteins that serve as molecular switches in many life processes in health and disease. But they contain a puzzling section of wobbly structure. A team from the University of Texas used the second-generation Anton system developed by D. E. Shaw Research and hosted at PSC to explore these proteins at longer time scales than possible with general-purpose supercomputers. The work revealed an ensemble of four structures that the protein rapidly switches between. This suggests the apparent disorder does serve a purpose, which may offer possible targets for drug therapies.
WHY IT’S IMPORTANT
Much of human health and sickness revolves around the proper functioning of our individual cells. A family of proteins called GTPases sits at the center of many cell activities. They serve as molecular “switches” in passing messages inside the cell, across the cell membrane. Scientists have linked dysfunction in GTPases with disorders as different as Alzheimer’s disease, cancer, depression, diabetes, and heart disease. Because of this, drugs targeting GTPases hold huge promise in treating many diseases.
There’s been a problem with this approach, though. When the first 3D structures of proteins emerged from X-ray studies, scientists thought that the amino acid chains that make up proteins would fold in a precise, even rigid way. But further experiments, particularly as computer simulations improved, showed that proteins moved, and much more than expected. GTPases proved to be an extreme version of that, with sections of amino acid chains that are intrinsically disordered. They really don’t have a folded structure that they settle on, and are constantly waving around.
“Traditionally, the flexible region [of GTPase] was thought to be just a passive linker to connect the soluble catalytic domain with the lipid-modified segment that inserts into the membrane. But over the years, mostly using molecular dynamic simulations, we are learning that the flexible region is not completely random. It actually might adopt some specific ensembles of conformations and interconvert among those. And that interconversion, we are learning, appears to be important for interacting with specific lipids in the plasma membrane.”
— Alemayehu Gorfe, University of Texas
GTPase proteins’ intrinsically disordered regions (IDRs) connect the part of the GTPase that carries out its function, and the part that anchors it to the cell membrane. At first, scientists thought that the IDR simply wiggled around, serving no function. But investigators started to suspect that the apparent disorder might somehow play a purpose in the protein’s function.
Graduate student Chase Hutchins, working in the University of Texas McGovern Medical School at Houston laboratory of advisor Alemayehu Gorfe, wanted to explore whether there was a method to these proteins’ madness. Whether, at longer time scales, these proteins’ IDRs formed short-lived folded structures that served a purpose. To achieve these time spans they turned to Anton, a special-purpose supercomputer for such molecular dynamics simulations that was designed and constructed by D. E. Shaw Research (DESRES). The second-generation Anton machine they used was made available to scientists without cost by DESRES, and was hosted at PSC with operational funding support by the National Institutes of Health. (DESRES recently replaced that Anton with a third-generation machine, which also receives operational funding from the NIH.)
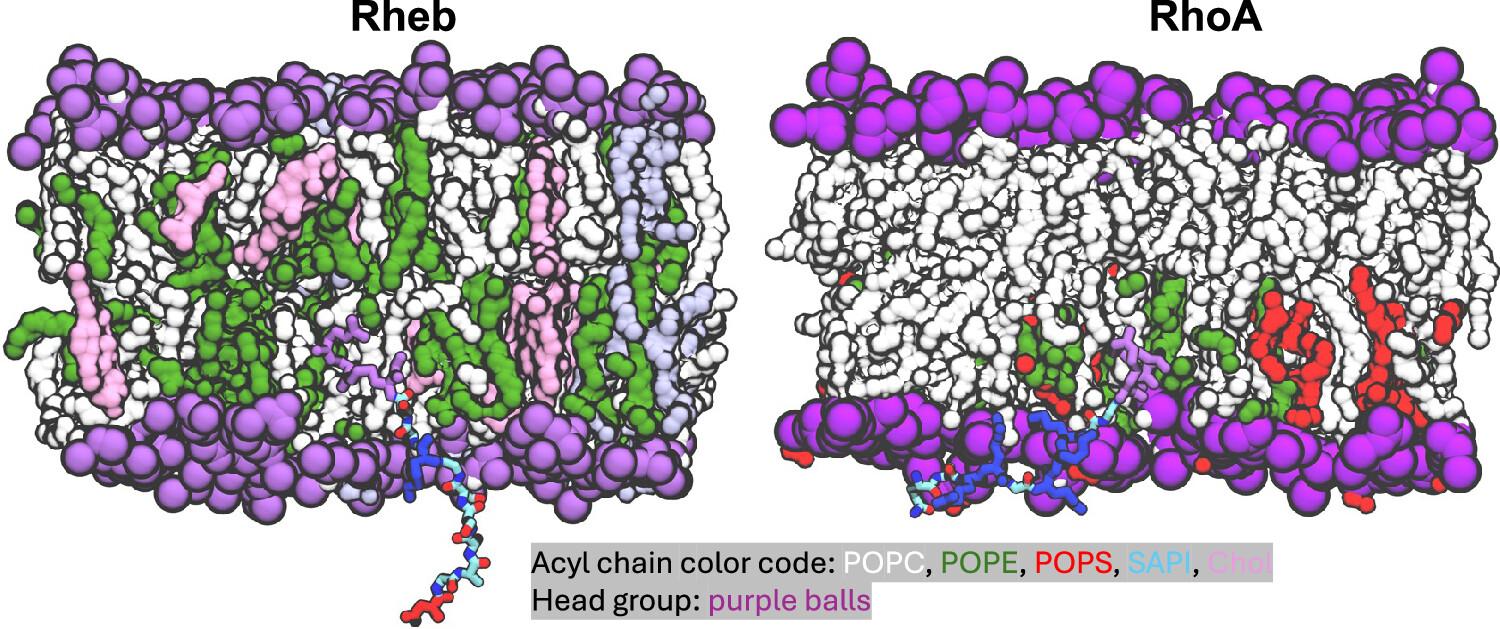
Snapshot of Rheb, a GTPase containing a disordered region (left) and RhoA, a GTPase that doesn’t have one, showing that region sticking out of the inner face of the cell membrane. From Fig. 3 of Intrinsically Disordered Membrane Anchors of Rheb, RhoA, and DiRas3 Small GTPases: Molecular Dynamics, Membrane Organization, and Interactions.
HOW PSC HELPED
The scientists would study three small GTPases, Rheb, RhoA, and DiRas3. Previous studies of cancer-associated GTPase Ras had offered tantalizing clues. But the Texas team wanted to see how more GTPases behaved, to get an understanding of the family’s common behaviors.
The team wanted to simulate how these proteins’ IDRs behaved over time spans that simply were not accessible with general-purpose supercomputers. The second-generation Anton at PSC specialized in these long, complex molecular simulations. The system could simulate large numbers of atoms in a complex protein molecule roughly 10 times faster than general-purpose systems. While short by everyday standards, a microsecond is an eternity for many computer simulations. Anton put microsecond simulations of large molecules within reach.
“What Anton does for us is one thing, and that is very critical. We want to run long time-scale simulations, because [we’re] talking about these complex mixtures of … lipids and proteins. … We can run microsecond-scale simulations very easily — get a microsecond, or a few microseconds, of data in a day. And that really meant all these [phenomena] are more and more accessible for us than [they would be] in most other systems.”
— Alemayehu Gorfe, University of Texas
When simulated for these longer timespans, the IDRs showed an intriguing property. The issue wasn’t that they didn’t have a structure. Instead, they had about four different structural ensembles, and kept switching between them. This ensemble structure may play a role in routing the GTPase from where it’s built to the site in the cell where it works. The four ensembles also provide possible binding sites in the protein that might be targets for drugs that act at different sites, fine-tuning the drug’s actions. The team reported their results in the Journal of Physical Chemistry B in June 2024.
The investigators would next like to understand the exact roles of the different structures and the protein’s dance between them. Again, an important question will be whether these roles, functions, and where they occur in the cell, provide targets to arrest disease processes with drugs. They plan to pursue these goals in part with the third-generation Anton, which became available at PSC in April 2025.
