Electrical Charge of Vaccine Particles May Lead to
Blood-clot side effect
Bridges-2 simulations suggest complication may stem from oppositely charged clot-forming protein sticking to engineered adenovirus
Despite the lifesaving success of the COVID-19 vaccines, very rare side effects have emerged. Vaccines engineered from the otherwise-mild adenovirus, for example, have been linked to blood clots. Scientists from Arizona State University, the Mayo Clinic, AstraZeneca and elsewhere have performed simulations on PSC’s Bridges-2 system that suggest simple electrical charge may make a protein involved in blood clot formation stick to particles of the AstraZeneca vaccine. The discovery will be the foundation of an effort to explain how the side effect happens and how the vaccine can be re-engineered to prevent it.
Why It’s Important
The COVID-19 vaccines have been a stunning success. They weren’t developed as quickly as many people think — SARS vaccines have been a subject of research since the first pandemic in 2003-2004. But they did introduce new vaccine technologies to the medical toolbox, in an unparalleled effort to test them and roll them out quickly and effectively that certainly saved many lives.
The vaccines, though, are not perfect. For example, those based on adenovirus have gotten some bad press because of a very rare, sometimes-dangerous side effect of blood-clot formation. Adenovirus COVID-19 vaccines include the AstraZeneca vaccine developed in Great Britain, the Johnson & Johnson vaccine in the U.S. and the Sputnik vaccine in Russia. Other COVID-19 vaccinations based on mRNA technology, such as the Pfizer-BioNTech and Moderna jabs, don’t contain adenovirus and can’t be directly related to these mechanisms.* Adenovirus is a type of virus whose natural form is known for mild, cold-like symptoms. Adenovirus-based vaccines use the virus to deliver SARS-CoV-2 virus genes that spur a response from the body’s immune system.
“[This side effect is] a very rare disorder, now, of course. But … when you tally the numbers of the vaccines … given all around the world, including in the U.S., that factors to a big number of potential patients. That’s why it remains important.”—Abhishek Singharoy, Arizona State University
Mind, this side effect is very rare. For a 55-year-old recipient, the risk of blood clots after a jab is only 4 in a million. That’s close to the chance of being struck by lightning. For someone younger, 25 years old, the risk is higher, at 11 in a million — but still very small. And we need to compare this risk to that of getting the virus. With the recent delta variant, the risk for the unvaccinated may have been as high as 1 in 20 to be hospitalized and 1 in 100 to die.
Of course, with billions of lifesaving vaccines being administered worldwide, even the adenovirus vaccines’ tiny side-effect risk winds up affecting a significant number of people. Doctors would like to know how these clots form, so they can predict which patients are at higher risk and route them to alternative vaccines. Scientists would also like to know how to re-engineer the vaccines to prevent clots. That’s why Abishek Singharoy of Arizona State University, working with scientists at the Mayo Clinic, AstraZeneca and elsewhere, turned to PSC’s NSF-funded Bridges-2 advanced research computer.
How PSC Helped
Scientists had discovered that a protein in the bloodstream, called platelet factor 4 (PF4), seemed to be sticking to the adenovirus-based vaccine particles. Since this protein plays a role in clot formation to heal wounds, the connection was an obvious target of research.
Singharoy and his colleagues knew it would be important to get a look at what was happening with the vaccine particles and PF4 at the molecular level. They decided to use a powerful combination of simulation on computers with “wet-lab” experiments. The simulations could generate ideas to be tested in the lab, and the lab results in turn could validate and correct the computer results. It’s a kind of scientific ping-pong match that has proved very successful in such medical research.
Using a computational method called Brownian dynamic simulation, Singharoy would get a prediction of how the vaccine particles interact with PF4 based on first principles, without making any assumptions about how the atoms in each molecule would act. But these simulations would require a lot of computation, as each one is a kind of alternative possibility for the motions of the molecules. A solid answer would require averaging hundreds of such simulations. Bridges-2’s “regular-memory” nodes offered this combination of power and parallel processing.
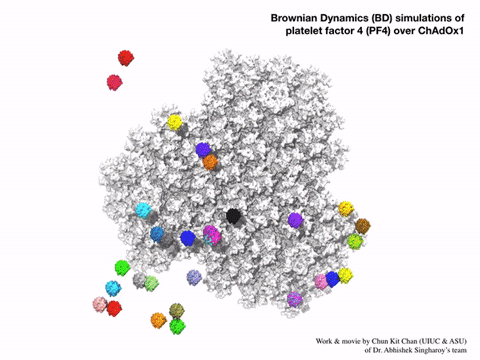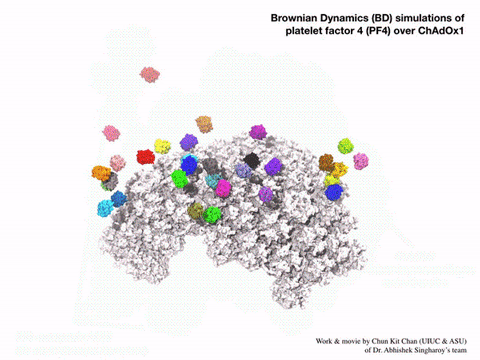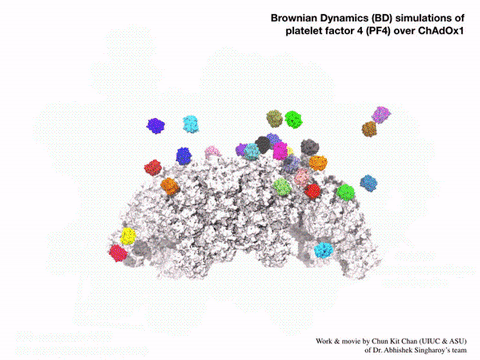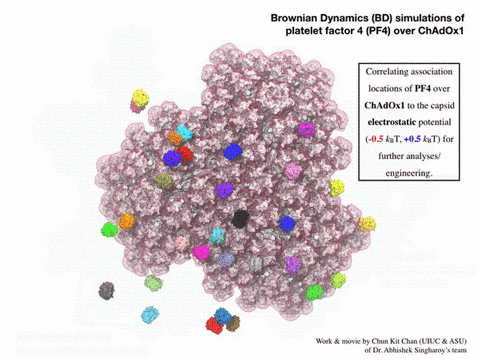
“We wanted statistically significant results, so we actually ended up running 400 parallel simulations, hundreds of parallel computations of the molecular docking of PF4 on the surface of the adenovirus vector … Now we’ve seen in molecular detail the structure of the vaccine and realized that the outer surface … is negatively charged, which was not something that you could guess [without the simulations].”—Abhishek Singharoy, Arizona State University
The Bridges-2 simulations offered a surprisingly simple explanation for PF4 sticking to the vaccine particles. The particles’ surfaces were highly negatively charged. PF4, by comparison, tends to have a positively charged surface. Negative and positive attracted, and PF4 stuck to the particles. Even more interesting, introducing the drug heparin, which prevents blood clots, into the simulations prevented PF4 from sticking to the engineered adenovirus.
Singharoy’s colleagues followed up with laboratory experiments involving the AstraZeneca vaccine and PF4. The strength of the interaction between the two in real life turned out to be exactly what the simulations predicted it would be, giving the collaborators confidence that their computations were on the right track. The team reported their results in the journal Science Advances in December, 2021.
A lot of questions remain to be answered. Charge attraction is a very plausible explanation for the clotting, but it would be only the first step in the clotting cascade. To fully understand the phenomenon, the scientists will need to follow the process from start to finish. Another question is why PF4 sticking to the vaccine particles causes dangerous clotting in only a small percentage of vaccine recipients, and how to re-engineer the vaccines to prevent the problem. Singharoy says that one avenue for future efforts will be to harness artificial intelligence — a particular strength of Bridges-2 — to explore possible improvements to the vaccine. Further work with the AstraZeneca vaccine, as well as possibly other adenovirus-based COVID-19 vaccines, will follow, making a very good family of vaccines even safer.
*Correction added 3/7/2022.