Picking Up the Beat
Anton 2 Helps Identify Unexpected Pathway for Irregular-Heartbeat Drugs
Irregular heartbeat—arrhythmia—causes a lot of sickness and death in the U.S. Some medications can treat arrhythmia, but they carry potentially serious side effects. Using Anton 2 at PSC a team from the University of California, Davis has performed previously impossible simulations of how the anti-arrhythmic drug lidocaine enters the cardiac sodium channel that initiates heart-muscle contraction. The results revealed an unexpected path of entry for the drug. The results may help scientists design better anti-arrythmia medications with fewer side effects.
Why It’s Important
Arrhythmia—irregular heartbeat—affects more than 2.3 million people in the U.S. alone, according to the Pharmacy Times. It can lead to heart failure, coronary artery disease, diabetes, hypertension, heart-valve disease and especially stroke. People with the most common type of arrhythmia have a risk of stroke that’s 4- to 5-times higher than someone without. Arrhythmia alone, without complications, can cost nearly $40,000 per year to treat per patient. Side effects of arrhythmia medications can increase that cost by almost $200,000.
“A big medical challenge we have is that we need more selective and more potent anti-arrhythmic drugs that would not have side effects. Our study was important in that it pinpointed some of the atomic details of the drug/cardiac sodium channel interactions.”—Vladimir Yarov-Yarovoy, UC Davis
That’s why postdoctoral fellow Phuong T. Nguyen and his advisor, Vladimir Yarov-Yarovoy, at the University of California, Davis, studied how lidocaine, a popular anti-arrhythmic drug, works. A better handle on the mode of action of these medications could help scientists design better drugs with fewer side effects. The UC Davis team turned to simulations of the drug as it interacts with the cardiac sodium channel, a protein in the cell membranes of heart tissue, using Anton 2 at PSC.
How PSC Helped
Anton 2 was the UC Davis scientists’ computer of choice for these simulations because of its specialized nature. Unlike general-purpose supercomputers, Anton 2 does only one thing. It simulates molecules as they move and interact. Because of this, the system can typically predict the interactions of large proteins and other biomolecules for longer simulated time periods than other supercomputers. Anton 2 is hosted at PSC thanks to manufacturer D.E. Shaw Research and operational funding by the National Institutes of Health.
The heart sodium channel is a protein that spans the outer membrane of heart muscle cells. The channel is critical to heart muscle activity. Heart muscle cells act as a kind of battery by taking advantage of uneven concentrations of sodium ions inside and outside the cells. When the sodium on either side of the cell membrane is uneven, the battery is charged. When certain nerve cells signal heart tissues, the sodium channel opens its pore to allow sodium ions to pass through. It then quickly inactivates, stopping the ion flow. This brief “open-inactivated” period collapses the sodium imbalance enough to trigger a signaling cascade involving other proteins. This cascade, in turn, causes the cell to contract. A proper open-inactivated period for the sodium channel orchestrates the organized contractions of a heartbeat. Disorganized contractions cause irregular heartbeat and improper pumping of blood—an arrhythmia. Lidocaine and some other anti-arrhythmic drugs work by stabilizing the sodium channel in an inactivated state. This prevents the channel from opening and stops the heart tissue from disorganized contractions.
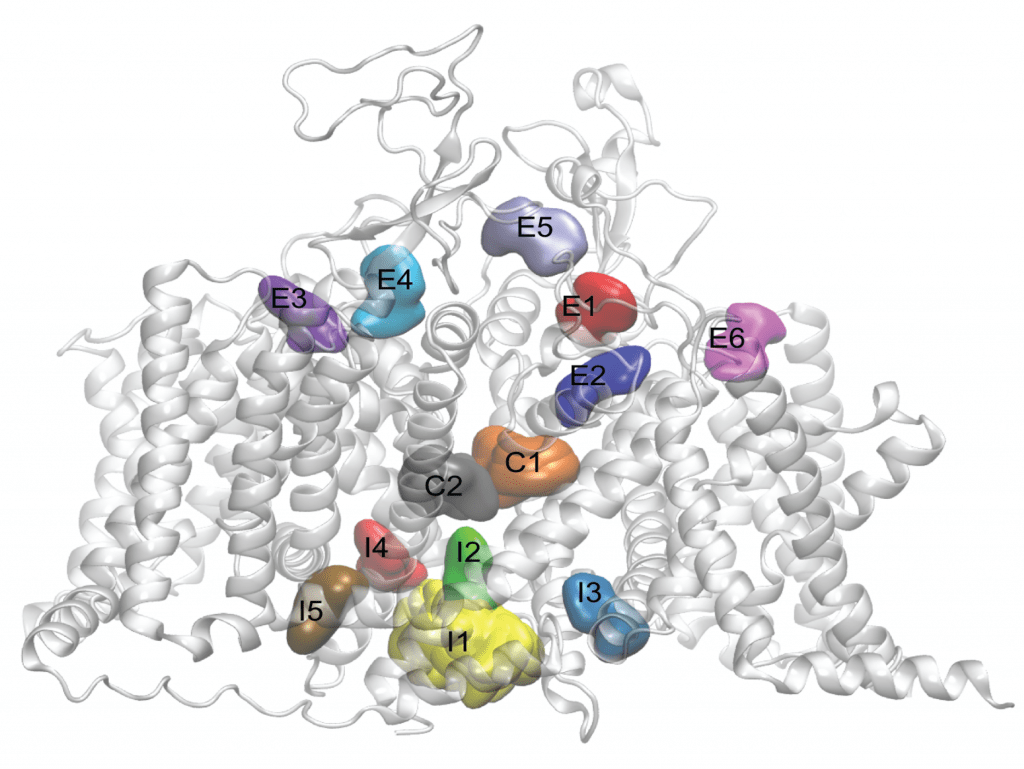
Image LEFT: Side view of the cardiac sodium channel, with neutral lidocaine binding sites represented as colored surfaces. From Nguyen PT et al. (2019) Structural basis for antiarrhythmic drug interactions with the human cardiac sodium channel. PNAS 116(80):2945-2954.
Nguyen and Yarov-Yarovoy’s goal was especially ambitious. They didn’t want to make assumptions about how lidocaine enters the sodium channel pore. They wanted simply to mix virtual versions of the lidocaine and sodium channel molecules in the computer. Using the known rules of molecular interactions, the computer would then calculate how they would behave. This approach had the advantage of allowing the scientists to observe the interaction without biasing the results based on previous researchers’ ideas. But it also required superior computational power.
The scientists would have to simulate the molecules for about 15 microseconds. That’s 15 millionths of a second. It may seem incredibly short, but in terms of computer power it’s a difficult problem. Thanks to Anton-2’s specialized hardware and software, the UC Davis team was able to simulate the neutral version of lidocaine in less than a day. The same simulation, they estimate, would have taken several years or more on a general-purpose supercomputer.
The team found that lidocaine could enter the cell in two ways. One was the expected route of through the channels pore gate. The second was by sliding between the protein and the surrounding membrane from the outside. While unpredicted, this method of entry made sense of some puzzling laboratory data on the channel. The team reported their results in the Proceedings of the National Academy of Sciences USA last year.
“There are some unexpected results. It is widely accepted that neutral drug molecules enter the sodium channel either from the intracellular pore gate or through the lipid membrane. Observing that lidocaine enters the channel from outside by climbing down between the channel’s outside wall and lipid membrane is very intriguing. ”—Phuong T. Nguyen, UC Davis
The UC Davis scientists would like to use the new information to design drugs that can affect the sodium channel more powerfully and with fewer side effects. One goal will be to repeat the simulations on the electrically charged “ionic” version of lidocaine. They’d like to learn how electrical charge affects the drug’s interaction with the sodium channel. Because of that charge, ionic lidocaine won’t be able to enter the cell membrane as easily as the neutral version and will require even more simulation time. The power provided by Anton 2—performing the simulations in one day instead of years—provides new insights that can be used to design more effective cardiac drugs.