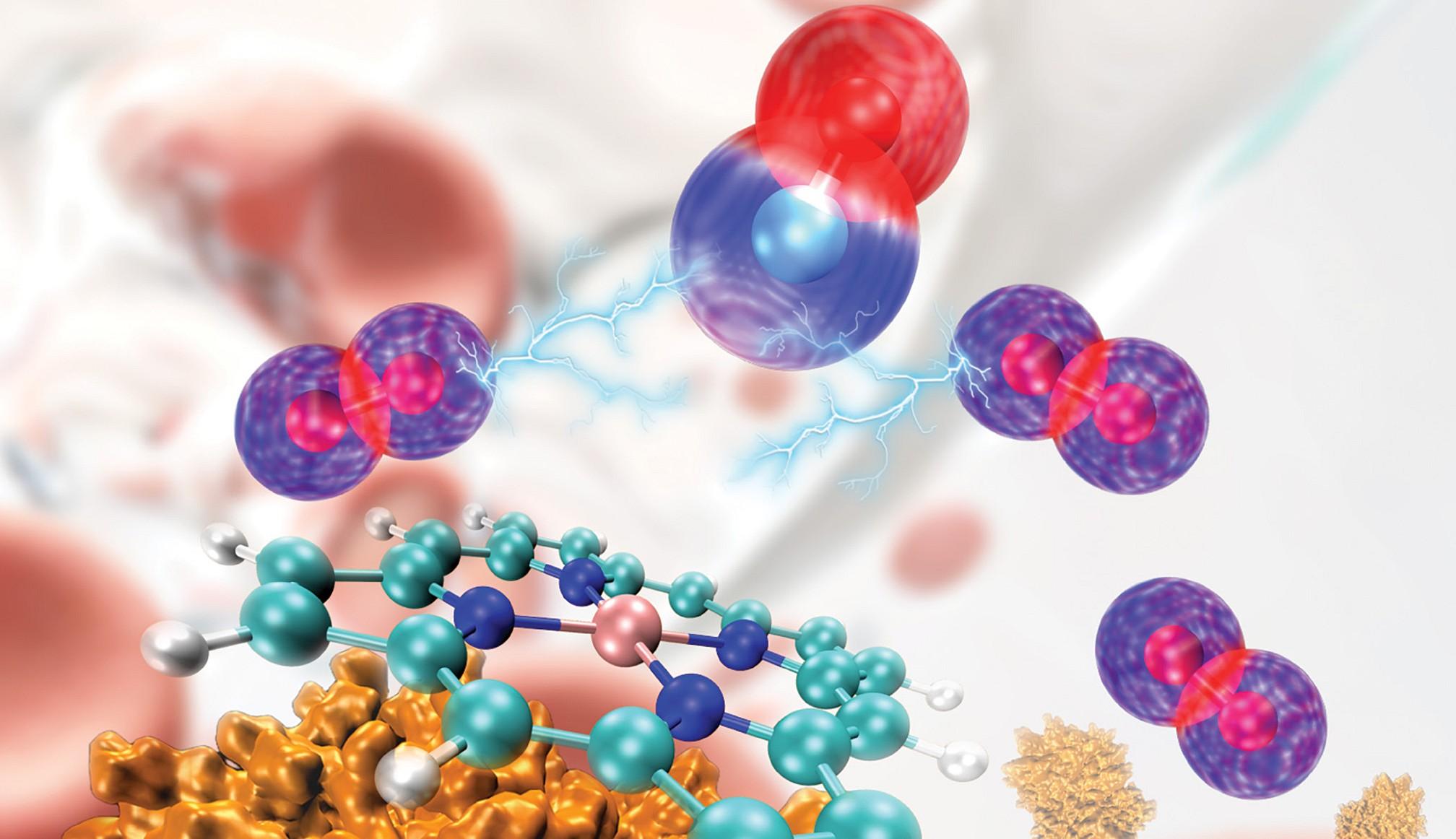
A Syracuse University team used Bridges-2 to correct earlier, mistaken simulations of the hemoglobin protein (lower left) and its oxygen-carrying heme group (center) and determine how carbon monoxide (blue and red spheres, top center) competes with oxygen (purple and red spheres). Cover image from the Journal of Chemical Theory and Computation, Vol. 20 Issue 10.
AI-Derived Parameters and Simulations on PSC’s Flagship Bridges-2 Offer Therapeutic Target for Carbon Monoxide Poisoning
Carbon monoxide has been a hazard to humans since we built our first fires. Current medical treatment for CO poisoning requires expensive and rare high-pressure oxygen chambers. Using PSC’s Bridges-2, a team from Syracuse University essentially “reinvented the wheel” for simulating the interactions of CO, oxygen, and the hemoglobin protein that carries oxygen in our bloodstreams. Their work corrected erroneous force field parameters that made initial simulations inaccurate, providing the first correct simulations showing how high-pressure oxygen can kick CO out of hemoglobin. It also pinpointed the first molecular target for development of future drugs that might displace CO more easily and at lower cost.
WHY IT’S IMPORTANT
Despite innovations like catalytic converters, electric vehicles, and detectors for the gas carbon monoxide, it remains a dangerous source of poisoning for humans. Exposure to structure fires, exhaust leaks in vehicles, and even normal work conditions in some industries like food service can put people at risk.
CO poisons us because it sticks to hemoglobin in our bloodstreams more tightly than oxygen does. This crowds out the oxygen that our blood can transport to vital tissues, suffocating us even when there’s plenty of O2 around.
We do have a very effective treatment for CO poisoning. Hyperbaric oxygen (HBO) therapy — placing someone in a chamber with high-pressure oxygen — gives our bloodstream enough oxygen to crowd back, displacing the CO and delivering oxygen to starved tissues. But HBO chambers are expensive. They also aren’t available in a lot of places. More accessible treatments for delivering more O2 to victims’ hemoglobin could save lives.
“I worked with my PhD student Mingrui Jiang to use the existing force field parameters to set up a molecular dynamics simulation to study the binding chemistry and environment of oxygen in hemoglobin, and how this might affect the binding affinity of carbon monoxide … Once we performed the [initial] simulations, we started to realize that an issue exited with the classic force field parameters. [By comparing to DFT calculations] the binding energy was not as expected. Also, the morphology of binding carbon monoxide to hemoglobin did not look right.”
— Zhao Qin, Syracuse University
A team led by Zhao Qin of Syracuse University wanted to better understand the interactions between the hemoglobin protein, CO, and oxygen using computer simulations. But before they could do that, they faced a problem. The previously accepted parameters describing the system weren’t accurately reproducing its real-life behavior. They quickly discovered they would need to re-evaluate those parameters, correcting them with a combination of first-principles simulation and AI prediction. They accomplished their goals using PSC’s flagship Bridges-2 supercomputer, thanks to an allocation in the NSF’s ACCESS network of supercomputing centers, in which PSC is a leading member.
HOW PSC HELPED
Real-world experiments had shown that a number of factors can help change the relationship between oxygen and CO in binding to hemoglobin. But the detailed interactions between the gas molecules within the protein can’t be directly measured. At the simplest level, it’s a matter of getting the O2 close enough to the protein’s binding side for the “good” gas to displace the CO. The parameters governing that distance were poorly known, though, and when the Syracuse scientists applied them they didn’t accurately predict the system’s real-life behavior.
To get the numbers right, Qin’s team would have to return to first principles, using density functional theory (DFT) to calculate how strongly each gas would bind, and how their atoms would be arranged. Then, they could use that information to feed a neural network AI to simplify the interactions into a force field — an approach that sums up the many factors into one. This would take far less computing power to simulate how the oxygen and CO would interact within the hemoglobin protein structure.
“Bridges-2 played a very important role in this project. This was a large-scale molecular dynamics simulation, and we also needed to conduct the DFT calculations … This high performance resource provided all the hardware we needed for our project. The massive numbers of CPUs in parallel greatly accelerated the work, taking a few months instead of a few years, if we had used our local workstation.”
— Zhao Qin, Syracuse University
Even with the force field simplification, the computations would be demanding. The ability of Bridges-2’s regular memory nodes to offer parallelization for DFT calculations, along with plenty of GPU nodes accelerating the MD simulations, allowed the team to break up the problem into more manageable chunks that could be crunched all at the same time, greatly speeding their calculations.
The resulting simulations offered a new window into how strongly CO binds to hemoglobin, and how close oxygen needs to get to kick it out. If the O2 molecule gets within 2.8 Angstroms — about one hundred-millionth of an inch — of the binding site in hemoglobin, it reduces the strength of the bond with CO by 50 percent. The scientists reported their results in the Journal of Chemical Theory and Computation in February 2024.
The result explains exactly at what point hyperbaric oxygen, which crowds the O2 in by force of pressure, manages to dislodge the poisonous CO. It also provides scientists with a target for drug design to create medications that can force oxygen in without hyperbaric oxygen. The Syracuse team wants to study these interactions in more detail, to better refine that target.
